Get patients started +
Download the Patient Enrollment Form to order ZUSDURI and access support services for eligible patients
Get patients startedZUSDURI delivered powerful, durable efficacy1
Not an actual patient.
Complete response (CR)

of patients achieved CR at 3 months with ZUSDURI
n=173/223 (95% CI: 72, 83)1*†
Duration of response (DoR)
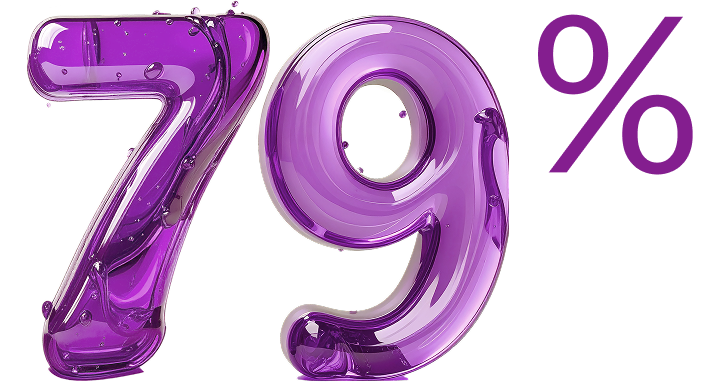
of patients who achieved CR remained in CR 12 months later (15 months from treatment initiation)1‡
*CR was assessed by cystoscopy, urine cytology, and for-cause biopsy at 3 months.2
†Based on observed DoR for 173 patients who had a CR.1
‡All patients were followed for a minimum of 15 months. Study is ongoing and further results will follow.1
Compelling outcomes, given:
55%
of patients had recurred within 12 months of prior episode1
84%
had multifocal disease3
0%
were treated with TURBT as part of this study treatment1
These data demonstrate that primary tumor ablation using ZUSDURI results in clinically meaningful CR rates, with durable responses observed 12 months later1
CI=confidence interval; TURBT=transurethral resection of bladder tumor.
References: 1. ZUSDURI [package insert]. Princeton, NJ: UroGen Pharma, Inc.; 2025. 2. Prasad SM, Shishkov D, Vladimirov Mihaylov N, et al. Primary chemoablation of recurrent low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102: a single-arm, open-label, phase 3 trial (ENVISION). J Urol. 2025;213(2):205-216. 3. Data on file. UroGen Pharma, Inc., Princeton, NJ.
Indications and Usage
ZUSDURI™ is indicated for the treatment of adult patients with recurrent low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC).
IMPORTANT SAFETY INFORMATION
Contraindications
ZUSDURI is contraindicated in patients with perforation of the bladder or in patients with prior hypersensitivity reactions to mitomycin or any component of the product.
Warnings and Precautions
Risks in Patients with Perforated Bladder
ZUSDURI may lead to systemic exposure to mitomycin and severe adverse reactions if administered to patients with a perforated bladder or to those in whom the integrity of the bladder mucosa has been compromised. Evaluate the bladder before the intravesical instillation of ZUSDURI and do not administer to patients with a perforated bladder or mucosal compromise until bladder integrity has been restored.
Embryo-Fetal Toxicity
Based on findings in animals and mechanism of action, ZUSDURI can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of mitomycin resulted in teratogenicity. Advise females of reproductive potential to use effective contraception during treatment with ZUSDURI and for 6 months following the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZUSDURI and for 3 months following the last dose.
Adverse Reactions
Common Adverse Reactions
The most common (≥10%) adverse reactions, including laboratory abnormalities, that occurred in patients treated with ZUSDURI were increased creatinine, increased potassium, dysuria, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, increased eosinophils, decreased lymphocytes, urinary tract infection, decreased neutrophils, and hematuria.
Additional Adverse Reactions Information
Clinically relevant adverse reactions occurring in <10% of patients who received ZUSDURI included increased urinary frequency, fatigue, urinary incontinence, urinary retention, urethral stenosis, genital pain, urinary urgency, genital edema, genital pruritus, genital rash, urethritis, acute kidney injury, balanoposthitis, and nocturia.
Use in Specific Populations
Lactation
Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with ZUSDURI and for 1 week following the last dose.
Preparation and Administration Information
ZUSDURI is to be administered by intravesical instillation only. Do not administer ZUSDURI by pyelocalyceal instillation or by any other route.
ZUSDURI must be prepared and administered by a healthcare provider. To ensure proper dosing, it is important to follow the preparation instructions found in the ZUSDURI Instructions for Pharmacy and administration instructions found in the ZUSDURI Instructions for Administration.
ZUSDURI may discolor urine to a violet to blue color following the instillation procedure. Advise patients for at least 24 hours post-instillation to avoid urine contact with skin, to void urine sitting on a toilet, and to flush the toilet several times after use. Advise patients to wash hands, perineum or glans with soap and water after each instillation procedure.
ZUSDURI is a hazardous drug. Follow applicable special handling and disposal procedures.
Please click here for Full Prescribing Information, Instructions for Pharmacy, and Instructions for Administration.